I have been grateful to have been a part of a small program (that needs to be a big program) that helps to include patients among the symposium attendees. They deserve a chance to hear the science. They deserve to be part of the conversation.
This year there were three Patient Fellows chosen through a blinded, competitive process. Their fellowships included the conference registration fees and modest lodging and some pretty cool bags.
 The scientific symposium runs from Friday through Sunday. In the week leading up to Friday, there is a melange of various meetings related to ALS that are not part of the symposium itself. One of those meetings is the "Ask the" session that I blogged about earlier this week. The reason that I chafe at the word experts in that context is exactly why I believe we need more people with ALS at the symposium. They are experts in ALS and know the outcomes that matter to them.
The scientific symposium runs from Friday through Sunday. In the week leading up to Friday, there is a melange of various meetings related to ALS that are not part of the symposium itself. One of those meetings is the "Ask the" session that I blogged about earlier this week. The reason that I chafe at the word experts in that context is exactly why I believe we need more people with ALS at the symposium. They are experts in ALS and know the outcomes that matter to them.The symposium is not webcast for people with ALS who cannot travel; therefore, I tweeted pretty constantly with frequent pictures of Powerpoint slides under the hashtag #alssymp .
Abstracts are available at
https://www.mndassociation.org/symposium/abstracts-online/
And so we begin.
Friday
After the welcomes, Dr. Mitsumoto kicked off with information on the Airlie House Guidelines which will finally be published in early 2019. Boy, it seems like they have taken a long time. He spoke mostly of process and not much of the specific content.
Princess Anne was an honored speaker, and I was honestly impressed. She gave a good talk and wasn't just reading pages. She also stayed to present some of the prizes that the symposium gives annually. Later she met with the local Scottish MND group and one of our Patient Fellows and his wife (from Edinburgh) were included. They both spoke higly of the her genuine interest and commitment to the cause.
Dr. Cryan gave a fascinating general talk about the microbiome and the nervous system (although without any specific references to ALS MND). It was educational and I was amazed at the promoted tweets that I received after tweeting some of his information. McDonalds needs to talk to its social media ad agency.
 There was a pseudo-debate on how we define ALS. It was interesting and more entertaining than a straight lecture. It established what we would hear as a continuing theme of ALS not being one disease and how clinical trial designs would have to be very different in the future.
There was a pseudo-debate on how we define ALS. It was interesting and more entertaining than a straight lecture. It established what we would hear as a continuing theme of ALS not being one disease and how clinical trial designs would have to be very different in the future. And my thoughts on disclosures:
Almost all of the panelists and speakers spoke to the need for biomarkers (as we have heard for 20 years). The idea of which biomarkers and what they are to show is making that discussion more specific.
Shaw presented on genes (a significant theme throughout the symposium). In one presentation, Dr. Hardiman mentioned that they don't see SOD1 in Ireland. Another person told me that they don't have C9 ALS in Korea. This certainly presents a challenge when we want an approval in one country to expedite an approval in another, and I'm very curious as to how genetics between Japan and US compare (thinking about edaravone). During one session, a researcher suggested that trial sponsors be required to run the full genome on trial participants so that better subgroup analyses could be performed.
And a related note from Shaw on the Ionis Biogen trial --
The symposium broke off from general sessions to tracks in the afternoon.
Throughout the researcher's presentations, we still see scant thanks to people with ALS and caregivers on their acknowledgement slides. I think some realized that they might be called out on social media for the omission, so we sometimes heard "of course, patients" slipped in at the end like "word peace." This is not a superficial issue to me. When they thank funders and colleagues and not the people who put up their lives and time to make their research happen, we've lost our way.
There was a session on decision support tools used in Australia that I missed that I wish I had seen.
Parmar spoke to trial designs in cancer Ph 3 trials (platform, basket, multi-arm, umbrella, etc) and how they can literally save time and money. One of the ALS neuroscientists gave the standard obj3ection, "but you can measure the tumor in cancer." Parmar really dismissed that and said that he doesn't see ALS trial design as something that much different than cancer. He gave specific examples of trials that was most helpful in understanding the tangible difference that innovative trials can make.
 VanEijk gave a fascinating study on the problems of genetic imbalance in ALS trials. This is what a friend with ALS was trying to tell them years ago. If the placebo group differs genetically from the treatment group, does that mess up your trial? If you don't have the right genetic mix in your trial, will your therapy go plop?
VanEijk gave a fascinating study on the problems of genetic imbalance in ALS trials. This is what a friend with ALS was trying to tell them years ago. If the placebo group differs genetically from the treatment group, does that mess up your trial? If you don't have the right genetic mix in your trial, will your therapy go plop?Brooks showed ibudilast results and mentioned muscle declines in the washout period. Do ethicists ever look at washout periods?
Miller presented results from the last NP001 trial and had a deer-in-headlights look when he got questions. This was another one of those overachieving placebo groups and the anticipated responders just couldn't keep up with those placebo folks. The company will not be living on and this one is finished. This is the therapy that was described in the book, Personal Trials by Jef Akst. I believe that any ALS trial designer needs to read it.
Saturday
Cytokinetics sponsored an early morning session with another pseudo-debate on whether trial inclusion criteria should be broad or narrow. Interesting discussion.
During the Q and A a Patient Fellow said that she didn't think she should have to choose between either NIV and a clinical trial. She eloquently brought out that we have standard-of-care issues in ALS trials. This is exactly why we should have more people with ALS and caregivers at the scientific symposium.
Dr. Genge hit some important points in her debate points --
We should not assume that just because there is no family history that a person with ALS does not have one of the known familial ALS genes.
Your inclusion criteria pick the placebo group as well as the treatment group. Include/exclude wisely.
By the end of the session it was clear that very specific inclusions that will permit a therapy to show if it works are desirable with some broad access programs that can also contribute information to the understanding of the therapy. I got a comment in that I think that every inclusion/exclusion criterion should have the scientific rational included. In the past they have seemed arbitrary and perhaps not well thought out. People will be more enthusiastic about trials if they understand why.
On to the track sessions, and Van Eijk gave a presentation on selection bias in ALS trials. I wondered if the inclusion criteria themselves might not contribute to that, too.
Chio presented on gender differences in ALS.
Shefner spoke to self-reported measures and how to make trials less burdensome to participants. He showed how some automated speech monitoring can be much more informative that the old ALSFRS-R. I wish we would stop talking and just do some of these things.
I missed the session on Speak Unique and maintaining spoken identity, but there was definitely a lot of buzz about the concept and the various concepts available now.
More followed on PROMS (patient reported outcome measures) and telehealth.
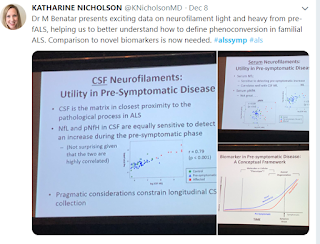
I switched over to a track digging deep into the genetics. At this point I was not in the room where Dr. Benetar gave the presentation that got the most overall buzz at the symposium -- hope for a presymptomatic biomarker.
In other presentations there were messages of needing to find the problem sooner than later in order to make treatments work. Alzheimer's was cited several times as a disease where much of the damage has started before it is recognized.
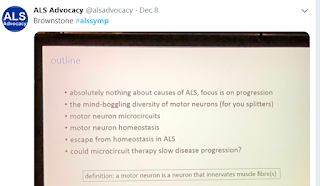
Brownstone gave a wonderful presentation on how motor neurons actually work (or don't work) in ALS progression.
I also saw a presentation on the MUNIX method for estimating the number of functioning motor neurons.
There was a session on old people (80+ years) with ALS and how the disease and management differ from younger people. This was a session where they could have used some old people with ALS to address their experiences rather than having researchers speculate on why they differ.
Sunday
There were presentations on metabolism. What we used to call Ben and Jerry's Therapy seems to be in order.
Thakore presented a controversial study on gastric tube placement and a lack of efficacy.
Jackson gave a presentation on the importance of NIV and the frustration that so many people with ALS are not "compliant." That's a loaded word. If you try something with a mask that bugs you and makes you feel worse, they call you "noncompliant." Blame the patient rather than the intervention.
There was a study to show superiority of SVC over FVC as a meaningful measure. I thought that was settled science several years ago, but they showed it again.
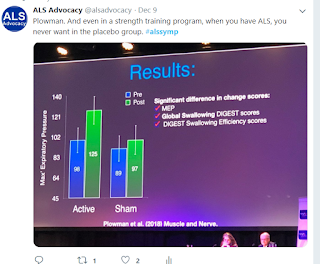
The star of the show as far as I was concerned was a study by Plowman of a respiratory strength training program that gave people with ALS improvements. That is far more impressive than any drug therapy we've seen.
At the end of the symposium, they usually have a slot for late-breaking news (and we hope something hopeful). This year it was results of the retigabine study which was not powered to show efficacy. More study needed. We first saw that as promising science four years ago.
Someday I would like to see how much each study cost included in the abstracts. Some of it seems priceless, and some of it, not so much.