This is the story of a couple of my friends and an investigational drug for ALS -- Neuraltus' NP001. It contains many pieces of ALS history that must not be forgotten. And they must never happen again.
Introduction -- It Started in 2010
_________
I met Rob and Ben online as a result of their participation on some ALS message boards. They were both really smart gentlemen with ALS. They were both scientists. Rob was an accomplished statistician who had professionally worked for General Motors on predicting car parts failures. He said it was excellent background for his second career in trying to outsmart ALS. Ben was a medical physicist. His expertise was in medical proton accelerators. He said that he worked on equipment that took cancer patients within inches of their lives so that they could live. He wanted the same right for himself. Rob and Ben were also young fathers with lovely families. We three all had dogs named Otis. And so the story begins. By time you finish reading this, you will know them, too.
It started in June of 2010. People interested in ALS science kibitz on the forum at als.net. The first inquiry about Neuraltus' NP001 came up. Did anyone know anything about it? The question was posed by Eric Valor, a young man with ALS and a gifted scientist. When he asks, people listen. And he was an essential force throughout the NP001 saga that followed. The images below are from conversations on the fora at als.net or patientslikeme.com.
The conversation began.
By August, Rob was really digging in. He and the others on the forum do this with everything that comes up in the news or literature, trying to figure out what the stuff is. Rob found evidence that it was related to a compound already marketed in Europe for other indications - WF10. It was some form of sodium chlorite.
The Phase I trial was a safety/dosing trial on the surface, but there was a placebo arm. Something else was going on. Every ALS trial is an efficacy trial, regardless of what the phase says it is. Maybe that was it. Or maybe they were looking for a biomarker. You just don't think of placebos as being essential in a safety/dosing trial.
Rob scoured the literature. That Phase I trial went quickly. By November they knew that NP001 was safe and a dose-dependent biomarker had been tracked. Interesting.
By December, Rob had uncovered some patent applications. The startling part was that as far back as 2005 the similar product, WF10, had been used in two people with ALS with good results. Five years earlier there was a reason to think that this could do something. Five years. Nobody pursued this in five years. That was another 600,000+ ALS deaths ago. You can see Rob's comment below.
Chapter I -- The 2010 Symposium
__________
Every December there is a global ALS symposium that attracts around 800 researchers and healthcare professionals. It has never encouraged people with ALS to attend, but that didn't stop Rob. It was in Orlando that year. He was interested in the science. Nothing would discourage him from attending. The most hyped new drug for ALS to this point was dexpramipexole. NP001 was still in the shadows.
Rob met researchers working on several clinical trial candidate drugs. He came home with a particular interest in NP001. It was based on data (albeit a very small sample) and science. And he wanted to find out if his patent research was correct.
Chapter II -- The Phase II Trial Starts
__________
Keep in mind that dexpramipexole Phase II trial enrollment was being hyped by a lot of organizations. NP001 was simply not on the radar. Rob was concerned about the NP001 exclusion criteria that would keep him out of that trial. He was quickly approaching 24 months post-onset, a traditional barrier to ALS trial participation.
NEALS (referenced in Rob's message above) is a large Clinical Research Organization (CRO) that is considered to be the leading such organization for ALS clinical trial design and administration. Neuraltus was using a different CRO.
People online were starting to get interested in NP001, and a man with ALS made a telling comment on a forum. There were no contingencies for access to NP001 after the trial or for those with ALS who did not qualify for the trial.
Yes, people with ALS can interact intelligently with scientists and business executives, yet they are never called to the table for input before things are designed. The importance of trial design will become even more apparent as you read on. In the meantime, Rob kept working trying to get the inclusion criteria for the NP001 trial expanded.
Rob was placing the investment of himself in medical research with NP001. He enrolled in the trial and didn't say too much publicly about his experience.
The schedule slippage began. Take a look at the last paragraph. Rob didn't say much at all publicly, but he told me that he was noticing things. He was still driving and one day realized that he was able to work some controls in the car that he had not been able to work before. ALS trials always try to determine if people are getting worse less quickly than they were before. Rob was actually seeing improvements. He didn't want to say much but was tracking data religiously and taking videos of what was happening. Ben had indicated that he was going to join the dexpramipexole trial. It seemed most promising to him. After some correspondence with Rob, he decided to try the NP001 trial even though it was less convenient for him. The two scientists wanted to be human lab rats with something that perhaps was actually doing something. It may not have been a long-term solution, but it was doing something. Ben enrolled and started NP001 infusions on June 20, 2011. And his tagline reflected his belief that the data must be captured publicly if the science is to advance. The trial was designed to require 21 weeks of infusions with a placebo, a low dose, or a high dose. Each participant would then be monitored every four weeks during a washout period to watch the activity of a proposed biomarker. No drug would be available during that washout period, and there would also be further monitoring through about 9 months with a final interview at 49 weeks. Chapter III -- Fill the Trial! __________ Ben was on a mission as you see below. Both Ben and Rob believed that if there were participants who were actually improving that the Data Safety Monitoring Board (DSMB) for the trial would step in and not withhold product from those who were experiencing improvements. Surely.
As the realization that the trial design wasn't ready for patients experiencing good things was sinking in, Rob asked people to reach out to Neuraltus.
Rob expressed frustration at NP001 not being on organizations' promoted trials lists.  They started speculating on possibilities for approval, yet they knew that the only thing they could control was filling that trial quickly. The reality of the trial design was sinking in. The biomarker was going to be tracked come hell or high water, regardless of what that meant for the people in the trial. Read Rob's last sentence below. And Ben questions whether we have developed an obsession with biomarkers and have lost sight of ethical ways to find them. And Rob agrees.
And the statistician Rob pleas with researchers to look at what is going on here. It's different.
Ben continued to fill seats in the trial. He traveled from Indiana to Lexington for his infusions. He searched for ALSA support group meetings in Lexington and showed up one night. The people there were not aware that in their own backyard there was a trial seeking volunteers. That's a telling indictment of the effectiveness of organizations at getting information to patients regarding clinical research. If they wonder why people don't enroll in trials, they should look in the mirror. Ben and Rob filled a trial in record time through their own efforts.
And Rob was giving interested people information that they were not getting from the trial sites.
And their efforts continued. And ALS organizations should have been embarrassed.
Both Ben and Rob were keeping meticulous records in their profiles at PatientsLikeMe.com. They were encouraging all in the trial to post their data, too.
And some things are just not captured by ALSFRS-R.
And they continued to recruit relentlessly.
There was still a confidence that the DSMB could and would see that some patients were getting actual improvements and would act. \  The term "Accelerated Approval" enters the conversation. Chapter IV -- The Horrible Premonition Sets In __________
The reality of an inflexible system is starting to set in to Rob who is approaching the end of his time on NP001. The DSMB won't learn about their improvements. There will be no decisions to modify the plan. And there is a long period staring at him when he will no access to the drug so that a biomarker can be tracked.
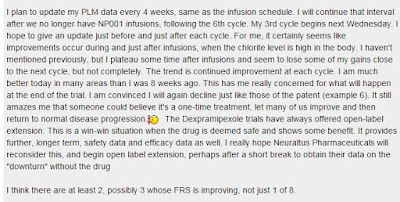
They are capturing and watching data. Statistician Rob has figured out some subgroups (high side effects, low side effects, no side effects), and responders seem to be in the high side effects group. Rob's words below are a terrible portent of what is to come -- "This has me really concerned for what will happen at the end of the trial. I am convinced that I wlll again decline, just like those of the patent..."
Ben comments on the trial design and his pessimism about what will happen next is setting in, too.
Ben will fulfill his commitment to the trial, but he is planning ahead. And his words in his last paragraph below about his work and cancer patients should stick with us all.
And Ben continued to monitor his grip strength, an outcome that this trial did not measure. And he was clearly measuring improvement.
And see Ben's words below. Surely the world would watch in horror as those in the study begin to decline again. Surely.
And Rob speculates and says that Neuraltus must lead. Surely they will. Surely. Rob speaks to what we already knew about the lessons from the old data on WF10. Is Neuraltus going to withhold drug to watch a biomarker crash while the trial participants crash? Why is this trial designed this way? Neuraltus knows the patients are going to crash. Why are they doing this?
And Rob notices that the patient advocacy organizations are conveniently failing to pay attention to their plight.
We hear more from Rob on his condition and the trial's endpoint selection. And again, Rob speaks to what is within the control of Neuraltus. Surely they will act. Surely. Chapter V -- Neuraltus and Others Fiddle, Patients Burn __________ Clearly the search for the worshipped biomarker is at odds with urgency and humane treatment of participants with this trial design. Rob and Ben finished their commitments to the trial. They endured the washout period and crashed, each losing the gains and wondering where they ended up relative to where they would have been without the trial. Neither posted much about how poorly they were doing without the NP001 while their data were monitored, but their emails reflected their frustrations and disappointment, not only with Neuraltus, but also with organizations that they thought would advocate on their behalf. Both tried some do-it-yourself NP001 options after that washout period. Ben was featured in the Wall Street Journal for his efforts. Both men tracked their data religiously at PatientsLikeMe so that others might learn. They weren't the only ones who wished the trial had been designed differently. I met a grandmother at the ALSA Advocacy Conference that year. She had terrible bulbar symptons. She said that she had been in the trial and had done so well, and since the washout period when they were observing her without NP001, she had just gotten so much worse. And there were others on the message boards who felt the same way. Below is Rob's ALS Functional Rating Scale R (ALSFRS R) chart from PatientsLikeMe. ALSFRS R is a summary measure that misses many details. You can see the uptick during the period when he was on NP001. There was an immediate and steep decline while Neuraltus watched his data decline relative to their biomarkers. And below is Rob's weight chart. Here is Ben's ALSFRS-R chart. ALSFRS-R wasn't granular enough to pick up many of his gains And Ben, too, had the precipitous weight loss. They did not have easy deaths. Rob died on September 10, 2012. ROBERT WAYNE TISON Ben died on August 15, 2013. Ben Harris, 46 Chapter VI -- So What? __________
This isn't meant to be about a particular drug. This is about real people and a process that failed them. This is about two beloved husbands and fathers who volunteered to advance the science who were stuck with a clinical trial design that was inhumane. This is about all those who weren't paying attention.
This must never happen again.
Every time we hear about the important search for biomarkers, we should ask whether that will be allowed to impede an efficacy trial.
Every time a drug developer proposes a Phase II trial on the cheap (and, yes, I realize that they are all expensive), we should ask whether it should wait until they can afford to treat the volunteers humanely.
Every time a person with ALS is handed informed-consent documents, he or she should ask, "What will happen to me if this stuff actually does something good?"
|