Yesterday I read the recent ALSA publication, "Evaluation of the ALS Association Grant Programs Executive Summary Report."
http://www.alsa.org/assets/pdfs/RTI-Report-FINAL.pdf?_ga=2.159412733.1999167149.1559913321-2099279597.1554299407
It's a long read, but it is also insightful on ALSA's claims to success in research investment since the 2014 ice bucket windfall. As I read the conjectures about how well things have gone, I also waited for the next paragraph that would cite the possible weaknesses or opportunities or threats to those successes.
What is the ultimate measure of success? We have the end goal of meaningful treatments, and we have failed. People are dying today from ALS just as they did before the ice fell. We really need the rest of the SWOT (strengths, weaknesses, opportunities, threats) analysis to help us change that most important outcome of all.
Following are from some thoughts and questions that I wrote in the margins of the report. I hope that some will be helpful to move the conversation forward to the even more important WOT part of the analysis.
Page 1. "...the sharp trajectory..."
It's important to realize that before the ice fell, there was a significant amount of pressure on ALSA to increase its research investments (which historically were pretty paltry). When the teacher grades on improvement, the student who was not performing well to begin with gets a big break.
Page 2. "...mirroring the upward trajectory of ... its research investments..."
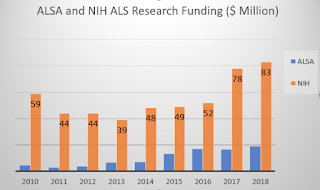
There is a lot of interesting analysis of NIH funding relative to ALSA research investments. Not to be lost in the conjecture is the fact that we have a causative vs. correlative puzzle here. Which comes first, the ALSA funding or the NIH funding, or perhaps one would have happened without any help from the other.
Here are the basics.
There's a chance that they are independent. During the years since 2010, ALSA has instructed advocates to ask for increased NIH funding for all diseases. Perhaps we did our jobs well in that regard, too.
Page 5
The new clinics are wonderful, but to the eye, we still have some large "clinic desserts" in the US. Does anyone know how many more people with ALS were put within 25 miles of a clinic by the clinic expansion?
Page 9
There's an interesting twist to their chart that shows the NIH funding that was gained by ALSA research grantees. I noticed that the NIH amounts on that chart were far larger than the NIH published totals for ALS. I thought perhaps they were getting non-ALS NIH funds, but the text in the report leads me to think that is not the explanation.
The ratio of researchers' NIH dollars to ALSA dollars is impressive. I wished they had a chart of the same data for the pre-ice years. When ALSA research investments were so small, that would make a small ALSA denominator with a possibly huge NIH numerator for some researchers.
The whole idea of ALSA grants seeding more NIH money seemed unsubstantiated when we didn't have a lead-lag analysis of the numbers. And the ALSA process for making grants is opaque to most of us. Is it a blinded selection, or are the grantmakers aware of the researcher and her or his potential for bringing in other funding sources? Again, it's hard to tell chickens from eggs.
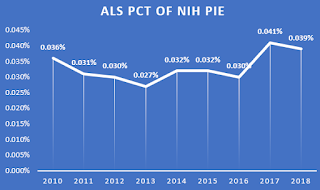
The most compelling data I think might simply come from looking at the ALS percent of the NIH total pie. We had been slipping or treading water for years. This chart shows that perhaps the NIH is seeing some opportunities and urgency to investing more in ALS. The percentage changes are small, but they are something.
Page 11
The publication analysis is interesting, but I wonder what it would look like if the analysis were done by institution and not invidivual. My experience is that there are many individual names on some papers who are included because of their position at an institution but have a very peripheral role in the immediate project.
Does the manner in which ALS MND Symposium abstracts are published skew any of the data?
It would also be helpful for ALSA to include publications for research projects in their research project database that is available to the public. Perhaps that could be a condition to a grant -- To supply ALSA for a non-paywall copy of publications for them to share.
Page 19 Research Grantee Survey
I think that there is a real possibility of "gratitude bias" in the responses. The happiest recipients who want to talk the most about their results are clearly more likely to influence the survey.
It would be interesting to get some feedback from people who were not provided with grants (realizing of course that they may have "sour grapes bias").
I'm glad nobody went to jail over the raffle.
Page 23, Figure 12 "...Identified New Biomarkers..."
Should that not indicate "Identified Potential New Biomarkers?"
Page 26, Table 5 "... 3 clinical trials either initiated by [the Association] and fully funded by [the Association]..."
Were any of the three trials interventional? That's a pretty impressive statement if they were.
Clinic information are interspersed in the document. Clinics are not necessarily ALS research arms, and I believe that anytime they are included in discussions about research investments, we should be very sure that they are providing correct, pertinent, and timely information to people with ALS and caregivers on clinical research opportunities. If they don't, then they don't deserve to be included in research accomplishments.
The ALSA Website Summary of the Summary
https://alsadotorg.wordpress.com/2019/06/05/understanding-the-impact-of-the-ice-bucket-challenge-on-the-als-associations-finances/#more-5820
I think that the accrual of net assets is indeed a legitimate concern. For many years before the ice, ALSA was a financially sound organization that ran responsibily on $20,000,000 or less in net assets. Yes, thanks to responsible stewardship and cooperative markets, that amound is considerably larger than it was for many years before 2014. We understand that there is a spend down, but it has taken five years of spend down and the assets are large and do not reflect the urgency that many of us feel.
The $2 billion quote for a new drug sets a perspective, but there are some facts that need to be added to that perspective --
- $2 billion does not insure a new drug
- Repurposed drugs like edaravone can be brought to market for a tiny fraction of that amount
- The cost to find therapies is as variable as ALS itself is.
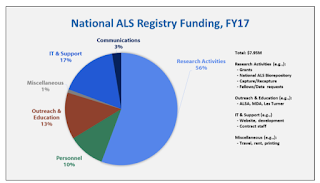
ALSA served over 20,000 people with ALS last year. That number makes a most expensive ALS research project, the CDC Registry, look terribly ineffective.
SWOT
We appreciate the successes and hope that all involved in this report will dig deeper with us all to figure out the weaknesses, opportunities, and threats. We must use precious resources in a smarter fight because by the measure that counts the most, people dying from ALS, we have not succeeded.
 Too often by the time a solution is proposed publicly, all some people can see are the potential design flaws. All some other people can see are potential benefits. The blinders are on. The "I'm right, you're wrong," public arguments begin.
Too often by the time a solution is proposed publicly, all some people can see are the potential design flaws. All some other people can see are potential benefits. The blinders are on. The "I'm right, you're wrong," public arguments begin.